We’re bringing together professionals who are passionate about advancing our understanding of psychedelic-assisted therapy in Canada with clinical research.
Join our Clinical Research CoP!
Our purpose is to cultivate a collaborative environment where clinicians, researchers, policy experts, and advocates can exchange best practices, discuss challenges, and align on quality, ethics, and protocols in psychedelic clinical research.

Clinical Research is part of the critical path to safe and legal access
Clinical research allows us to objectively measure the effects of psychedelics in humans —and is essential for future regulatory approval and healthcare system integration. Clinical trials pave the way for safe, well-informed, and legal therapeutic use.
Who Should Participate:
- Professionals looking to learn more about clinical research
- Researchers with experience in clinical research who can share perspectives
- Advocates passionate about advancing safe and legal access to psychedelic-assisted therapy
- Professionals looking to connect and foster peer support regarding psychedelic clinical research in Canada
- Patients with lived experience participating in psychedelic clinical research
- Anyone else involved in psychedelic research
Our goal is to build a community that is:
- A vibrant place to discuss what works and what doesn’t in conducting psychedelic studies, and to ask questions about related topics (e.g., protocol creation);
- A place to identify gaps in knowledge and practice, and to co-create resources to address them; and
- A one-stop “shop” for resources and expertise related to psychedelic clinical research in Canada.
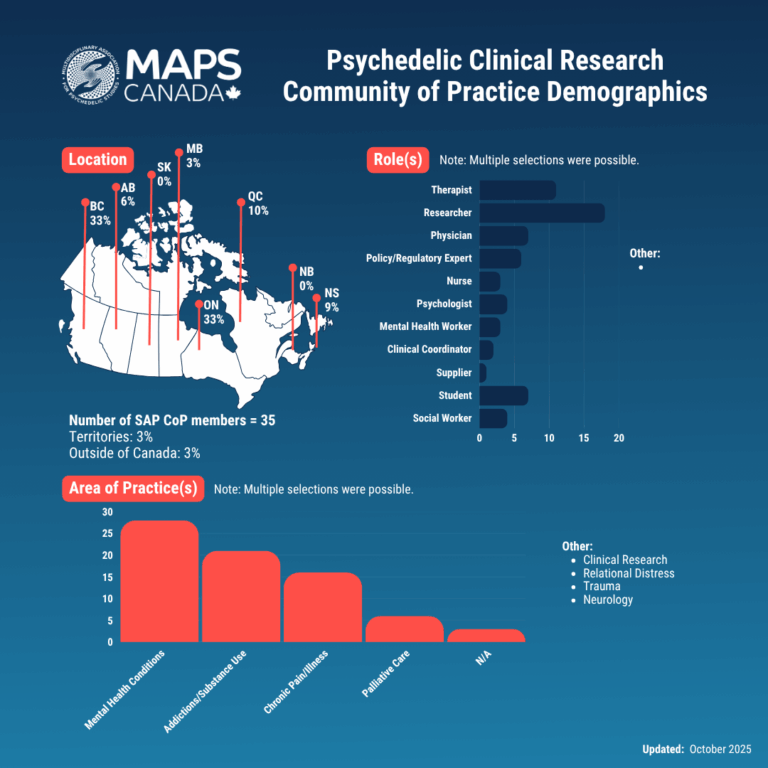
Sign up to become a Community of Practice member and join us for bi-monthly Zoom meetings to connect and collaborate! We will use this platform to discuss clinical trials as a treatment pathway, ongoing research, and barriers and ethics around Canadian psychedelic research.
Questions? Feel free to contact us at: pa-cop@mapscanada.org.
